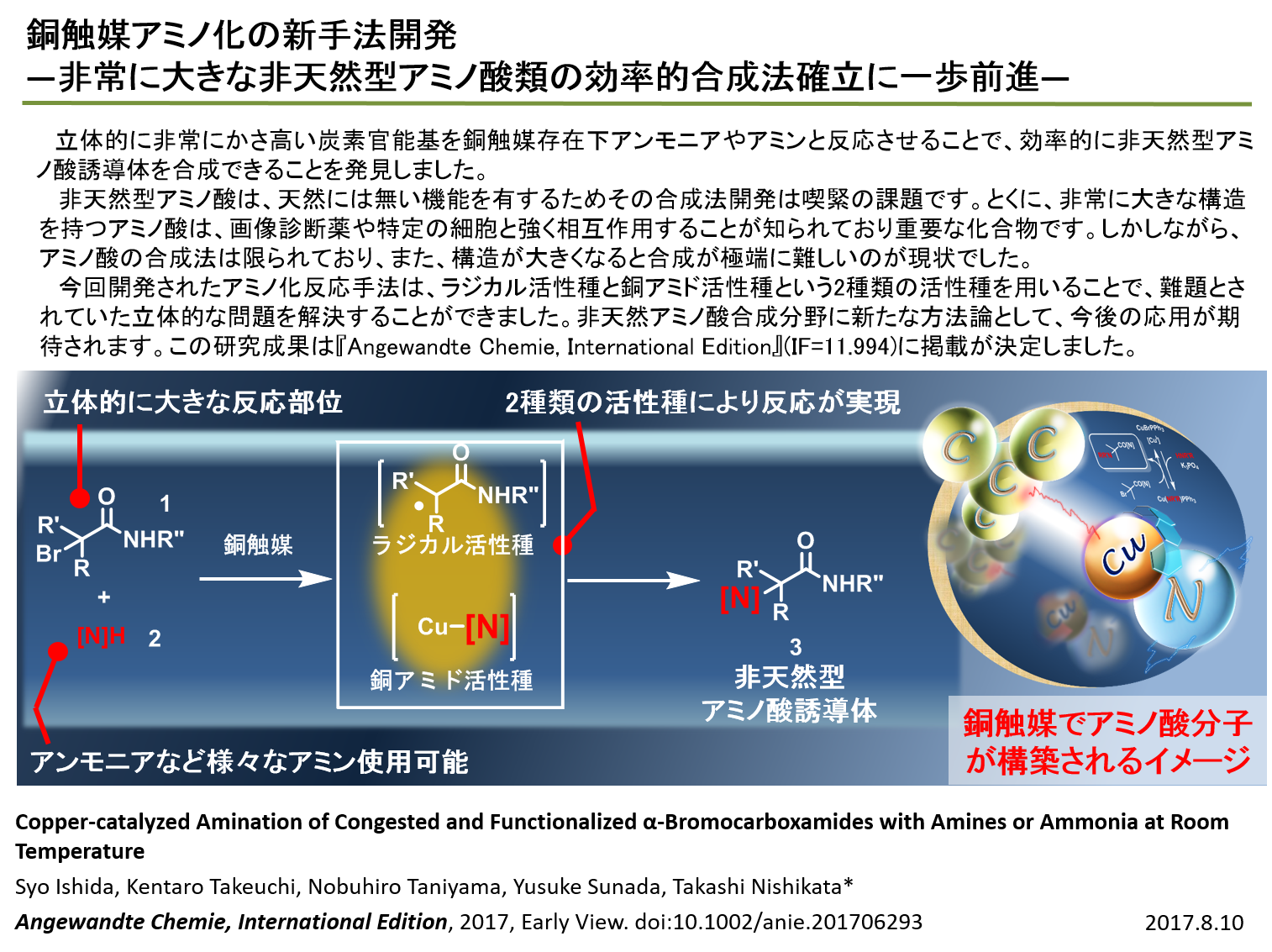
 |
| Amination: There are several reports on the synthesis of alkylamines, but most of
the reported methods are not suitable for the synthesis of hindered amines.
In this research, we found that a copper catalyst is effective for the
formation of congested C-N bonds at room temperature. Control experiments
revealed that a copper amide is a key intermediate. Moreover, when a chiral
amine was used, a quaternary carbon stereogenic center was created with
good selectivity. |
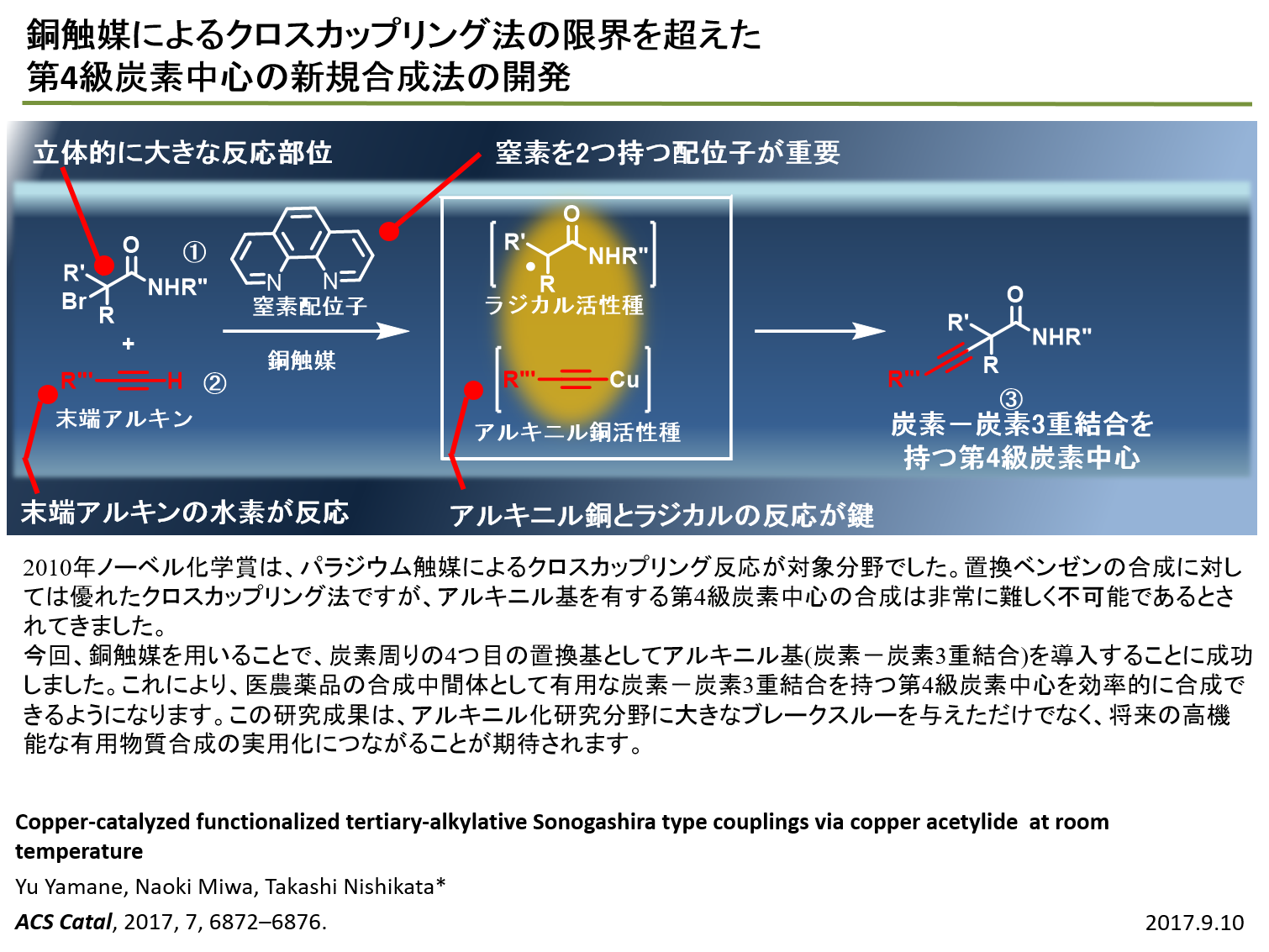 |
| Sonogashira:There are several reports on Sonogashira couplings, but most of the reported
reactions have employed aryl or alkenyl halides as coupling partners. Therefore,
Sonogashira coupling is unsuitable for alkyl loadings, especially tertiary
alkyl groups. In this research, we found that a copper catalyst is effective
for a reaction between a terminal alkyne and an α-bromocarbonyl compound
to form a quaternary carbon having alkynyl group at room temperature. Control
experiments revealed that a copper acetylide is a key intermediate. |
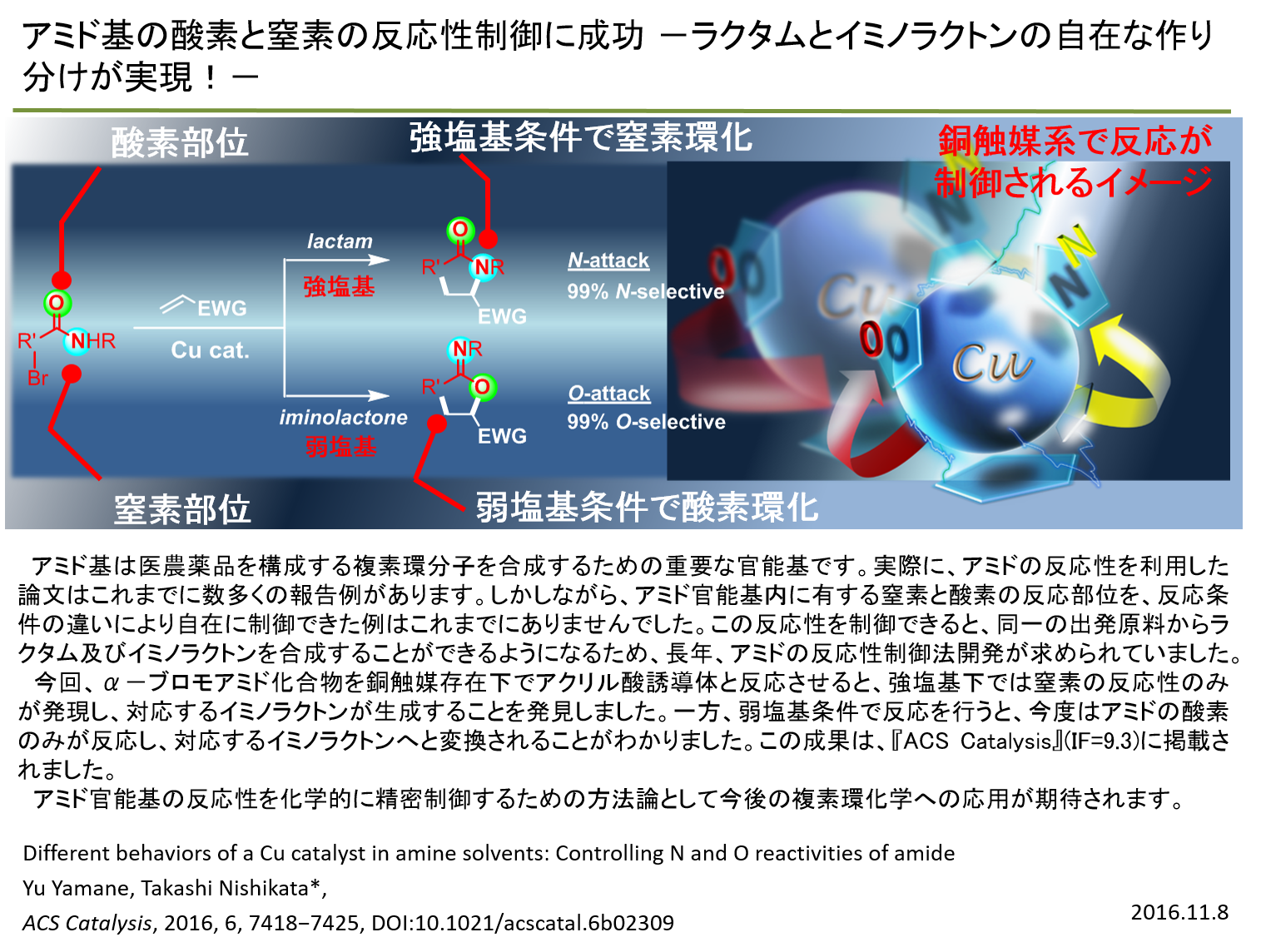 |
| Amide reactivities: Controlling the reactivity of the nitrogen or oxygen nucleophile of an amide group to form C−N or C−O bonds by tuning reaction conditions is one of the most challenging issues in the use of amides in organic synthesis. Both nucleophiles in the amide group can individually participate in reactions, and most reactions employ a substrate-controlled methodology to achieve selectivity. However, in the reaction of α-bromoamides and acrylates, we successfully controlled the reactivity of the nitrogen or oxygen nucleophile of the amide group to afford a lactam via carboamidation or an iminolactone via carbooxygenation, using a copper catalyst system with an appropriate base. |
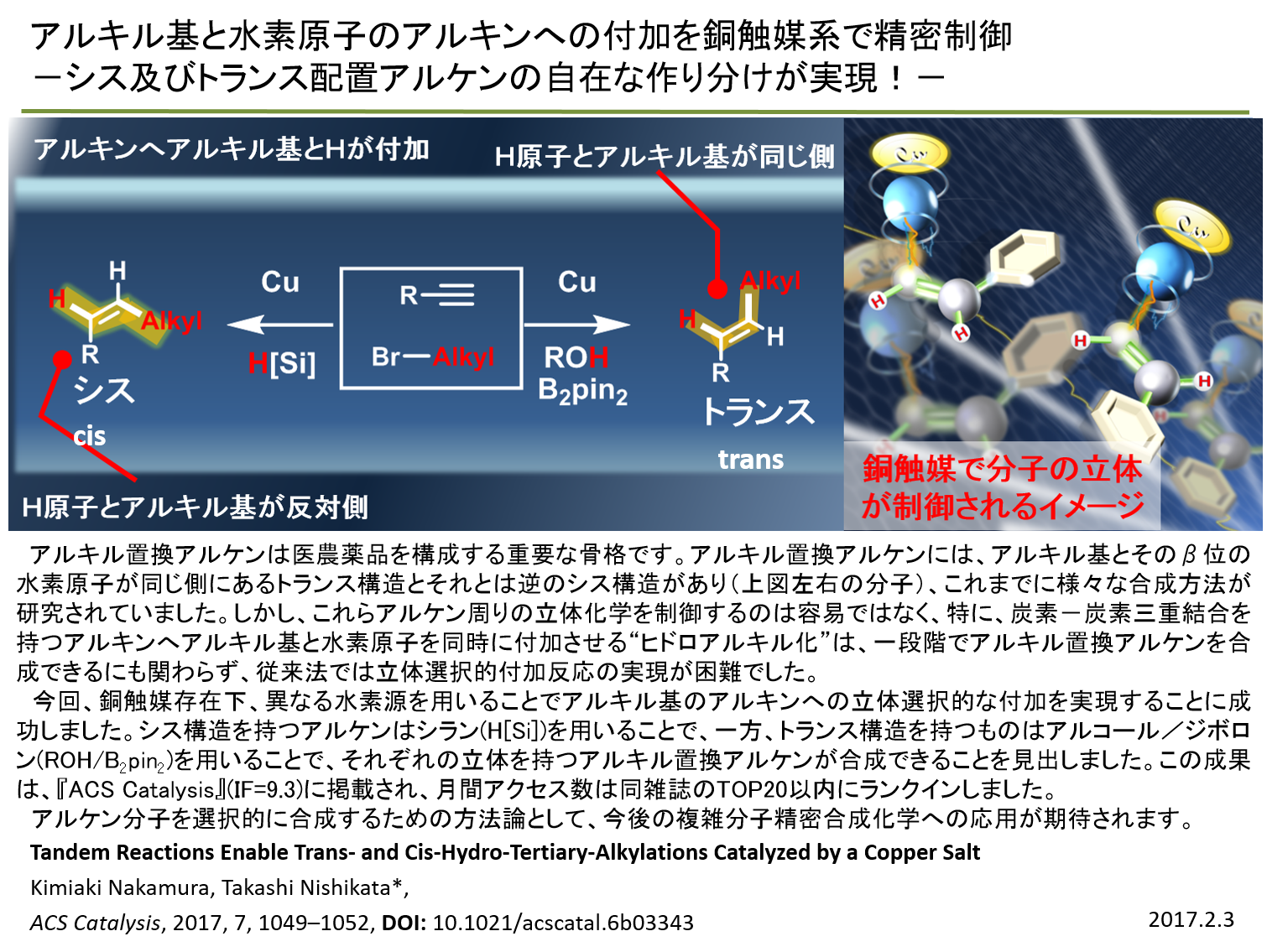 |
| Additions: A methodology to synthesize trans- and cisalkenes via well-controlled
hydroalkylation of alkyl radicals to alkynes is reported. α-Bromocarbonyl
compounds are useful alkyl radical precursors in the presence of Cu(I)
catalysts. Under copper catalyst conditions and in the presence of silane
or alcohol/B2pin2, trans- and cis-hydroalkylation occurred with excellent
stereoselectivities. The judicious choice of additives allowed for this
stereodivergence, giving selective access to the trans-alkylated alkenes
with HSiTMS3 and cis-alkylated alkenes with t-BuOH/B2pin2 in good yields
with selectivities. |
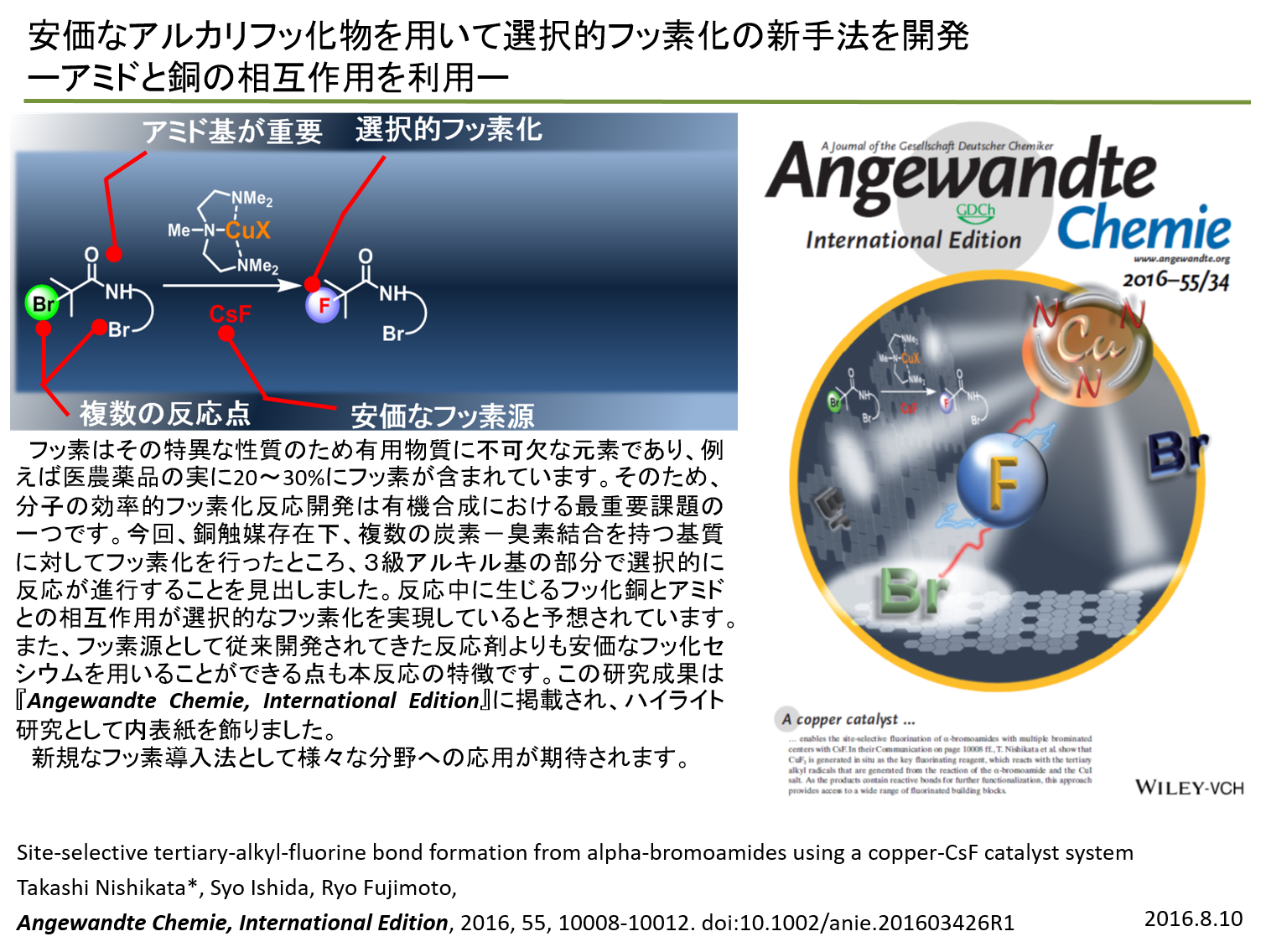 |
| Fluorinations: A copper-catalyzed site-selective fluorination of a-bromoamides possessing
multiple reaction sites, such as primary and secondary alkyl¢Br bonds,
using inexpensive CsF is reported. Tertiary alkyl¢F bonds, which are very
difficult to synthesize, can be formed by this fluorination reaction with
the aid of an amide group. Control experiments revealed that in situ generated
CuF2 is a key fluorinating reagent that reacts with the tertiary alkyl
radicals generated by the reaction between an a-bromocarbonyl compound
and a copper(I) salt. |
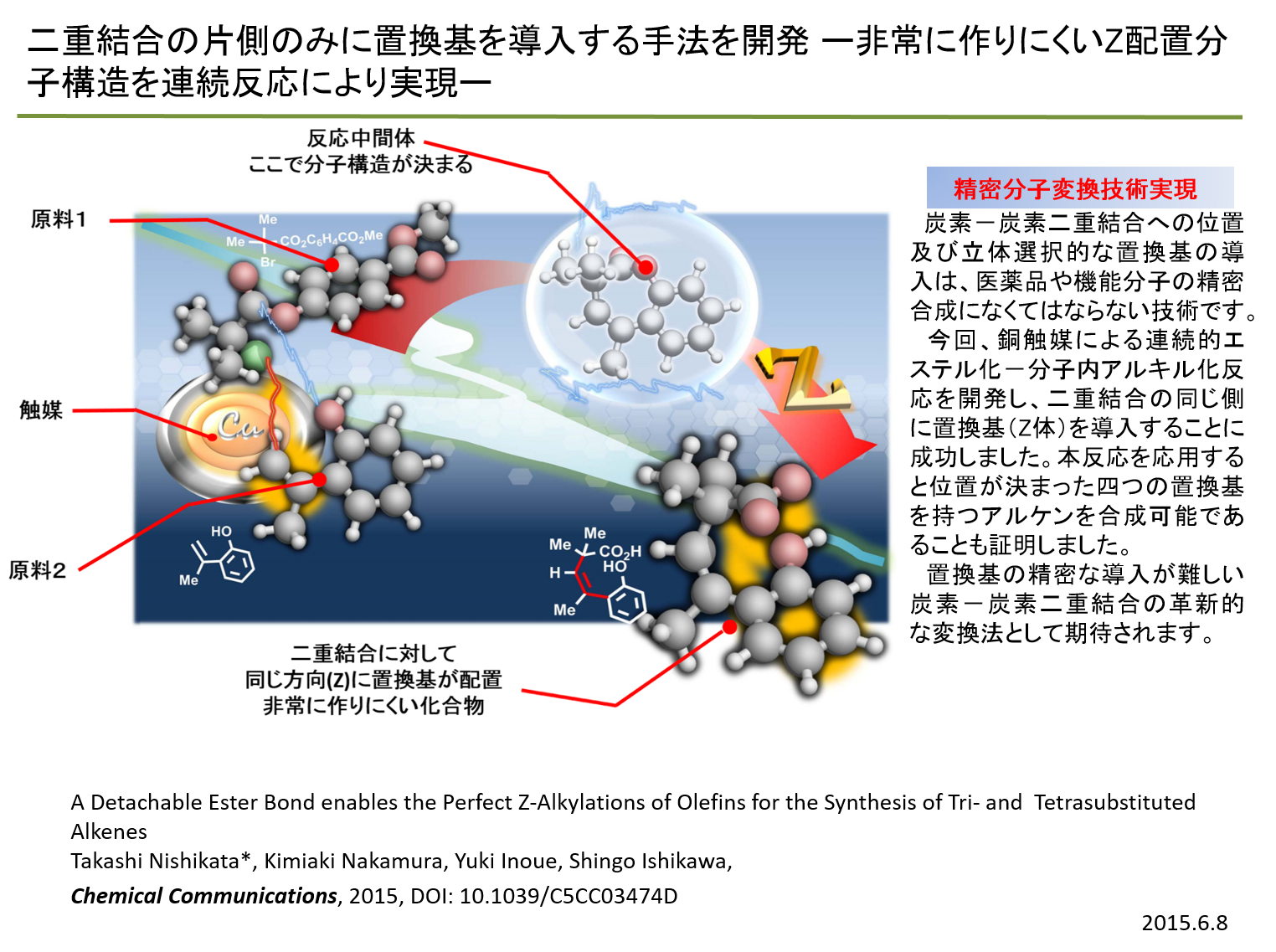 |
| Z-selective: 2-Vinyl-substituted phenol and an alpha-bromoester undergo a tandem esterification–alkylation
reaction in the presence of a Cu–amine catalyst system to produce benzene-fused
lactone. Z-Alkylated styrene is obtained after hydrolysis of the lactone
with perfect selectivity. The simple protocol developed in this work opens
a new avenue in the multi-substitution chemistry of alkenes. |






